Cambridge 7th to 9th September

Presenting Author:
Oliver Alderman
<oalderman@matsdev.com>
article posted 28 Feb 2015
Oliver Alderman
Oliver Alderman currently works as Research Scientist at Materials Development Inc.,
and is a Visiting Scientist at Argonne National Laboratory. He obtained an MPhys
degree in Physics in 2009, and a PhD in 2013, both at the University of Warwick.
His PhD studies included the use of x-ray and neutron diffraction and nuclear magnetic
resonance spectroscopy to study the structure of oxide glasses and related materials.
Current topics of research include the study of highly refractory melts and the temperature
dependent structure of liquids during glass formation or redox, primarily through the use
of the aerodynamic levitation and laser heating approach, combined with x-ray and neutron scattering and computational modelling.

Temperature dependent structure of molten borates:
testing the predictions of thermodynamic models
Oliver Alderman1,2,* Chris Benmore2, Lawrie Skinner7, Marek Liska8, Jan Machácek9, Guillaume Ferlat3, Axelle Baroni3,4,5,Mathieu Salanne4, Matthieu Micoulaut5,Anthony Tamalonis1, Alex Lin1,6, Richard Weber1,2
 Here we use high-energy synchrotron x-ray diffraction to monitor the structure of
aerodynamically levitated liquid B2O3,
and sodium and calcium borate melts as a
function of T for direct comparison to thermodynamic model predictions.
Here we use high-energy synchrotron x-ray diffraction to monitor the structure of
aerodynamically levitated liquid B2O3,
and sodium and calcium borate melts as a
function of T for direct comparison to thermodynamic model predictions.
Thermodynamic models, such as the model of ideal associated solutions, have shown quantitative agreement with measurements for many room-temperature properties of glasses.
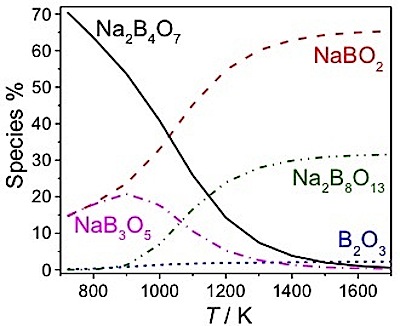 On the other hand, very few, if any, tests of the structural
predictions for melts and their temperature (T) dependencies have been made.
We find quantitative agreement in the boron-oxygen coordination number, CN,
which may increase, decrease or not change at all with T, depending upon the
melt composition.
On the other hand, very few, if any, tests of the structural
predictions for melts and their temperature (T) dependencies have been made.
We find quantitative agreement in the boron-oxygen coordination number, CN,
which may increase, decrease or not change at all with T, depending upon the
melt composition.
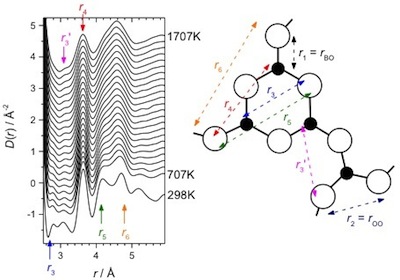 We argue that using bond length, BL, as a proxy for CN leads
to more accurate results in the present case, and this method can be corrected for
BL thermal expansion using our direct measurement of this quantity for pure liquid
B2O3. For liquid B2O3
we show that the diffraction data are qualitatively sensitive
to the presence of boroxol rings, and the temperature dependent structural modifications
are consistent with boroxol ring dissolution, a phenomenon supported by previous
Raman scattering studies.
We argue that using bond length, BL, as a proxy for CN leads
to more accurate results in the present case, and this method can be corrected for
BL thermal expansion using our direct measurement of this quantity for pure liquid
B2O3. For liquid B2O3
we show that the diffraction data are qualitatively sensitive
to the presence of boroxol rings, and the temperature dependent structural modifications
are consistent with boroxol ring dissolution, a phenomenon supported by previous
Raman scattering studies.
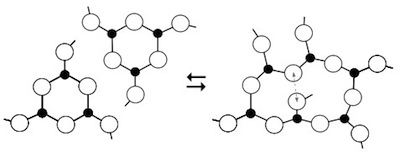 As well as demonstrating a deep connection between
crystalline and melt chemistries, the present results suggest that predictive power may
be greatly enhanced by combining thermodynamic modelling with modern versions
of constraint theory.
As well as demonstrating a deep connection between
crystalline and melt chemistries, the present results suggest that predictive power may
be greatly enhanced by combining thermodynamic modelling with modern versions
of constraint theory.
Authors' institutions:
1. Materials Development, Inc., Arlington Heights, IL 60004, USA
2. X-Ray Science Division, Advanced Photon Source, Argonne National Laboratory, Argonne, IL 60439, USA
3. Sorbonne Universités, UPMC Université Paris 06, UMR 7590, IMPMC, F-75005 Paris, France
4. Sorbonne Universités, UPMC Université Paris 06, UMR 8234, PHENIX, F-75005 Paris, France
5. Sorbonne Universités, UPMC Université Paris 06, UMR 7600, LPTMC, F-75005 Paris, France
6. Department of Materials Science and Engineering, McCormick School of Engineering and Applied Science, 2220 Campus Drive, Northwestern University, Evanston, IL 60208, USA
7. Mineral Physics Institute, Stony Brook University, Stony Brook, New York, NY 11794-2100, USA
8. Vitrum Laugaricio, Joint Glass Center of Institute of Inorganic Chemistry of SAS, Alexander Dubcek University of Trencín and RONA, j.s.c., Studentská 2, Trencín, 911 50, Slovak Republic
9. Department of Glass and Ceramics, Institute of Chemical Technology Prague, Technická 5, 166 28 Prague, Czech Republic
