Cambridge 7th to 9th September

Presenting Author:
Laura Swansbury
<las52@kent.ac.uk>
article posted 24 Feb 2015
Laura Swansbury
After studying for an undergraduate masters degree in physics at the University of Kent, Laura wanted to carry on studying glasses using molecular dynamics. She was lucky to get offered an EPSRC studentship which allowed her to start a PhD in September 2014 working with her previous supervisor Dr. Gavin Mountjoy. Laura?s research involves modelling the role of chlorine in bioactive glasses.

Molecular dynamics modelling of ZnCl2 glass
Laura Swansbury
School of Physical Sciences, University of Kent, Canterbury CT2 7NH, UK
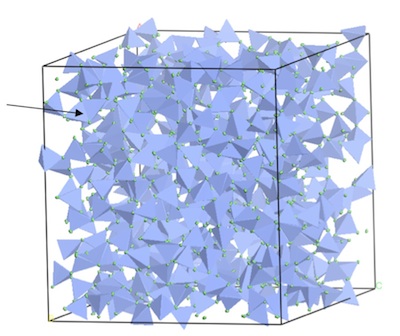 Figure 1: molecular dynamics model of ZnCl2 glass (blue represents Zn, light green represents Cl and arrows show edge-sharing tetrahedra).
Figure 1: molecular dynamics model of ZnCl2 glass (blue represents Zn, light green represents Cl and arrows show edge-sharing tetrahedra).
Reference:
[1] A. Zeidler, P.S. Salmon, R.A. Martin, T. Usuki,* P.E. Mason, G.J. Cuello, S. Kohara, and H.E. Fischer (2010) Phys. Rev. B 82, 104208 - "Structure of liquid and glassy ZnCl2".
