Cambridge 7th to 9th September

Presenting Author:
Josef Hormes
<hormes@physik.uni-bonn.de>
article posted 4 Mar 2015
Josef Hormes

The corrosion of stained glass:
a chemical analysis using synchrotron radiation based X-ray absorption
and X-ray fluorescence spectroscopy
Josef Hormes (1,2)*, Wantana Klysubun(3), Markus Kleine(4)
The corrosion of glass is a very complex process as it depends on external conditions (micro-climate, pollution) as well as on the composition of the glass (color, content of potassium etc.). While the basic mechanisms of the degradation process seem to be understood, there are still several open questions that should be answered for finding the "best" method for restoration (e.g. cleaning) and conservation of historic glass.
 Because of its special properties (high intensity, collimation, continuous spectrum from
the Infra-red to the hard X-ray region) synchrotron radiation based techniques allow the
detailed and (at least in principle) non-destructive chemical characterization of the
weathering crust but also of the "pristine glass": synchrotron-radiation excited X-ray
fluorescence (SR-XRF) allows the (semi-quantitative) determination of the elemental
composition of the samples incl. minor and trace elements and also "low-Z-elements"
such as S, Si, Mg and Na. X-ray absorption near edge structure (XANES) spectroscopy
provides detailed information about the chemical speciation of the elements of interest
and thus direct information about corrosion processes. In most cases, for example, the
valence state of the atomic species of interest can be determined and conclusions can be
drawn on the chemical environment of these atoms by comparing the XANES spectra of
the "unknown compound" to those of appropriate reference compounds
(fingerprinting method). By focussing the X-ray beam, both techniques can be applied
2d-spatially resolved and by focussing also the fluorescence radiation on the detector
side also depth profiles can be measured.
Because of its special properties (high intensity, collimation, continuous spectrum from
the Infra-red to the hard X-ray region) synchrotron radiation based techniques allow the
detailed and (at least in principle) non-destructive chemical characterization of the
weathering crust but also of the "pristine glass": synchrotron-radiation excited X-ray
fluorescence (SR-XRF) allows the (semi-quantitative) determination of the elemental
composition of the samples incl. minor and trace elements and also "low-Z-elements"
such as S, Si, Mg and Na. X-ray absorption near edge structure (XANES) spectroscopy
provides detailed information about the chemical speciation of the elements of interest
and thus direct information about corrosion processes. In most cases, for example, the
valence state of the atomic species of interest can be determined and conclusions can be
drawn on the chemical environment of these atoms by comparing the XANES spectra of
the "unknown compound" to those of appropriate reference compounds
(fingerprinting method). By focussing the X-ray beam, both techniques can be applied
2d-spatially resolved and by focussing also the fluorescence radiation on the detector
side also depth profiles can be measured.
In this contribution, we present several examples for the applications of SR-XRF and XANES experiments for the analysis of corrosion products from glasses from different production times and different origins: some glasses from the Cathedral in Paderborn (~ 12th century) several glasses of different color from window 116 of the Cathedral in Chartres (~ 1230 A.D.) and glass from one window from the Cathedral in Sevilla (16th century). For the glasses from Chartres only the mechanically removed corrosion products were measured and for the glasses from Paderborn the glasses with the corrosion layer. Parts of the glass from the Seville Cathedral were cleaned using three different techniques:
1. mechanically using a spatula,
2. "chemically" using EDTA,
3. mechanically using a diamond file.
The corrosion products that incur using techniques 1 and 3 were collected and included into the investigation so that the corroded glass, the glass cleaned with one of the 3 techniques, the "pristine glass" and the corrosion products could be measured separately.
 Experiments were carried out at the beamline 8 at the Synchrotron Light Research
Institute (SLRI) in Nakhon Ratchasima (Thailand) and at the DCM beamline of the
Center for Advanced Microstructure and Devices (CAMD) at Louisiana State
University in Baton Rouge (USA). Fluorescence for XRF measurements was excited
using monochromatic radiation of 10 keV and detected using either a 13-element
Ge-detector or a Si-drift detector. For the assignment of elements and a rough
calibration of fluorescence intensities NIST standard glass sample NIST 610
was measured. XANES spectra were recorded using double-crystal X-ray
monochromators equipped with crystals suitable for the corresponding X-ray
range - in most cases InSb(111) for lower and Ge(220) for higher energies.
Most XANES experiments were carried out in fluorescence mode with the set-up
used for the SR-XRF experiments.
Experiments were carried out at the beamline 8 at the Synchrotron Light Research
Institute (SLRI) in Nakhon Ratchasima (Thailand) and at the DCM beamline of the
Center for Advanced Microstructure and Devices (CAMD) at Louisiana State
University in Baton Rouge (USA). Fluorescence for XRF measurements was excited
using monochromatic radiation of 10 keV and detected using either a 13-element
Ge-detector or a Si-drift detector. For the assignment of elements and a rough
calibration of fluorescence intensities NIST standard glass sample NIST 610
was measured. XANES spectra were recorded using double-crystal X-ray
monochromators equipped with crystals suitable for the corresponding X-ray
range - in most cases InSb(111) for lower and Ge(220) for higher energies.
Most XANES experiments were carried out in fluorescence mode with the set-up
used for the SR-XRF experiments.
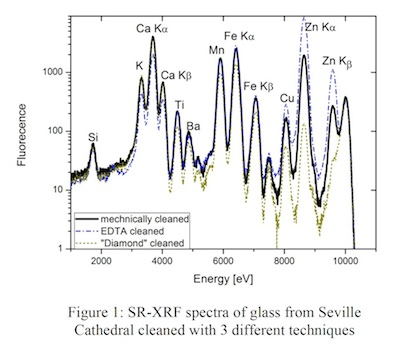
As an example for the SR-XRF experiments, Figure 1 shows the SR-XRF spectra of parts from the front side of the "Seville glass" cleaned with the 3 different techniques. By looking, for example, at the Zn Kalpha line it is obvious that the different techniques remove significantly different amounts from this element that is obviously not part of the original glass, whereas an effective removal of the corrosion layer increases the intensity e.g. for the Ca Kalpha indicating that Ca is a part of the original "pristine" glass.
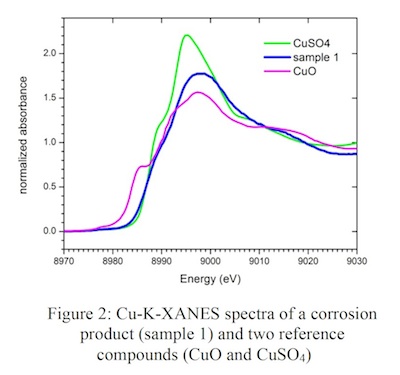
Figure 2 shows as an example for the XANES spectra, the Cu-K-XANES spectrum of a powdery corrosion product from a glass sample from the Cathedral in Chartres the in comparison with the spectra of two reference compounds (CuO and CuSO4). Just by the visual comparison of the spectra it is obvious that Cu in the sample is not a sulfate as often discussed in the literature.
Authors' institutions:
1 Institute of Physics, Bonn University, Nussallee 12, D-53115 Bonn, Germany;
2 Center for Advanced Microstructures and Devices, Louisiana State University, Baton Rouge, USA;
3 Synchrotron Light Research Institute, Nakhon Ratchasima, Thailand;
4Glasmalerei Peters GmbH, Paderborn, Germany;
*Hormes@LSU.edu
