Cambridge 7th to 9th September

Presenting Author:
Ekarat Meechoowas
<ekarat@dss.go.th>
article posted 04 Apr 2015
Ekarat Meechoowas
Working at Glass Expert Laboratory, Physics and Engineering Program, Department of Science Service
Graduated Doctor of Science (dr.rer.nat) in glass chemistry from Otto-Schott Institute, Friedrich-Schiller-Universität Jena 2010.
Responsible for glass research and testing to support the glass industries in Thailand. The current project includes improving glass melting efficiency, color controlling and improving properties of glass.

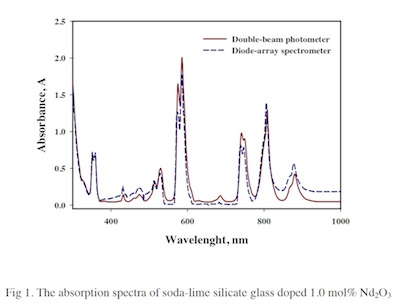
Spectroscopic studies of neodymium doped soda-lime silicate glass
Ekarat Meechoowas*, Parida Jampeeruang, Kanit Tapasa and Tepiwan Jitwatcharakomol
Glass Expert Laboratory, Physic and Engineering Program, Department of Science Service, Ratchathewi, Bangkok, Thailand 10400
In order to prepare the glass filter to use as a control sample for the spectroscopic measurement, the different amount of neodymium oxide (Nd2O3) doped soda-lime silicate (74SiO2-16Na2O-10CaO) glasses were prepared with 0.125 0.25 0.5 and 1.0 (mol%). The glasses were melted twice in platinum crucibles at 1500 ºC to avoid bubbles and annealed at 550 ºC. The thermal and optical properties of glasses were investigated. The spectra of these glasses were characterized by two different UV/Vis spectrophotometers, namely double-beam photometer and diode-array spectrometer. The results showed the intensity of absorption peak depending on the concentration and the similar peak positions for both spectrophotometers. The glasses were kept in desiccators for 6 months and measured once a week to verify the repeatability. The found percentage of relative standard deviation was less than 5%. The glasses with high neodymium concentration (0.5 and 1.0 mol%) presented strong and shape absorption peaks and were suitable for being control samples. The statistic calculation showed insignificant changes of spectroscopic overtime. The soda-lime silicate glass doped neodymium is the alternative way to produce the self-control sample for the spectrophotometer.